Peer Reviewed
A Systematic Approach to Evaluating and Managing QTc Prolongation in Patients Undergoing Methadone Agonist Treatment for Opioid Use Disorder
AUTHOR:
Moronkeji Fagbemi, MD
AFFILIATIONS:
Unit Chief Inpatient Detoxification Unit, BronxCare Health System, Bronx, New York
Department of Medicine, Icahn School Medicine at Mount Sinai, New York, New York
CITATION:
Fagbemi M. A systematic approach to managing QTc prolongation in patients undergoing methadone agonist treatment for opioid use disorder: evaluation and risk stratification. Consultant. 2021;61(7):e1-e5. doi:10.25270/con.2021.03.00006
Received September 8, 2020. Accepted December 22, 2020. Published online March 5, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Moronkeji Fagbemi, MD, Life Recovery Center, BronxCare Hospital Center, 1285 Fulton Avenue, Bronx, NY, 10456 (mfagbemi@Bronxcare.org)
Opioid use disorder (OUD) remains a significant cause of morbidity and mortality in the United States and throughout the world. Fewer than 10% of people with OUD receive effective treatment. Thus, OUD screenings and current treatment optimization is important. In 1972, the US Food and Drug Administration (FDA) approved methadone, a long-acting synthetic opioid, and a significant means of treating OUD. However, it can cause QT interval (QTc) prolongation, which can lead to torsade de pointes (TdP) development. This can increase the risk of sudden cardiac death by 4-fold. Hence, health care providers need a thorough and systematic approach for treating patients with methadone for OUD.1,2
Understanding the Opioid Problem
In the United States, more than 750,000 people had died from 1999 to 2018 from drug overdose, with almost two-thirds of these deaths involving opioids.3 Worldwide, about 500,000 deaths are attributable to drug use, with more than 70% of these deaths related to opioids and more than 30% of these deaths caused by overdose.4 Deaths from drug overdose continue to contribute to mortality in the United States with rate of drug overdose deaths involving synthetic opioids other than methadone (drugs such as fentanyl, fentanyl analogs, and tramadol) increased by 10% from year 2017 to 2018.5 In 2018, 46,802 overdose deaths involved opioids.6 This accounted for 69.5% of all drug overdose deaths in 2018, and 67.0% of opioid overdoses had involved synthetic opioids (excluding methadone).6
Patients with OUD have a more than 4-fold increased risk of premature death from accidental overdose, trauma, suicide, and infectious diseases than the general population.7 Risky behaviors associated with OUD increase the risk of exposure to HIV, viral hepatitis, and other infectious agents. This often occurs through contact with infected blood or bodily fluids from sharing syringes and injection paraphernalia or through unprotected sexual contact.8 According to the Council of Economic Advisers, the economic burden of the opioid crisis was $696 billion in 2018 alone.9 The value of lost lives, increased health care, substance abuse treatment, increase in criminal justice costs, and reduction in productivity predominately drive these costs.9 The COVID-19 pandemic causing strain on the health care system and preventive measures such as social distancing, in addition to economic downturn, has Introduced new risks to people impacted by substance use disorder, as well as series of new challenges related to treatment and recovery.3
Evaluation Begins with a Proper Diagnosis
Opioids include substances derived from the opium poppy (i.e., morphine, codeine), semi-synthetic opioids (i.e., heroin, hydrocodone, oxycodone, and hydromorphone), and synthetic analogues (i.e., methadone, fentanyl) with similar effects.4,10 The Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association defines OUD as a “pattern of opioid use leading to clinically significant impairment or distress, as manifested by at least 2 or more” of the 11 criteria for OUD diagnosis, that occur within a 12-month period.11 The presence of 6 or more criteria denotes severe opioid use disorder.11 Ongoing maintenance medications such methadone or buprenorphine in combination with psychosocial treatment appropriate for a patient’s needs is the standard of care for treating OUD.12
OUD treatment improves a patient’s health and facilitates participation in a rehabilitative program, while decreasing the risk of adverse outcomes associated with abstinence from opioids.13
Understanding Risk Factors for QTc Prolongation
Methadone is a long-acting synthetic mµ-opioid agonist. It has a half-life of 15 to 40 hours and is metabolized by the isoenzymes CYP3A4 and CYP2D6 of the hepatic cytochrome P-450 system. Methadone can cause serious adverse effects such as TdP and respiratory depression. Thus, the FDA and manufacturer include a black box warning regarding QTc interval prolongation and serious arrhythmia (i.e., TdP).2,14,15
Drug-induced arrhythmia often results from multiple factors (Table 1), including electrolyte abnormalities such as hypokalemia, hypomagnesemia, hypocalcemia; structural heart disease; hepatic cytochrome P450 inhibitors; female sex; and genetic predisposition, manifested by a prolonged QTc at baseline.16,17 The prolonged QT syndrome is characterized by the lengthening of the cardiac repolarization caused by alterations in the transmembrane potassium, sodium, and calcium currents. This prolongation of cardiac action potentials manifests on electrocardiography (EKG) scans as lengthening of the QTc, which serves as a forerunner to TdP and can cause sudden cardiac death.18
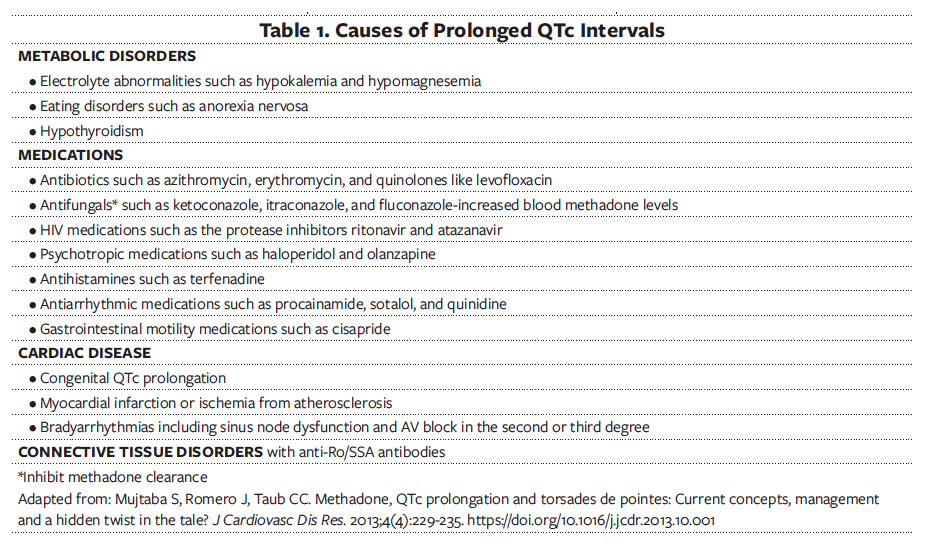
Methadone is an inhibitor of the cardiac ion channel KCNH2 and causes QTc prolongation in a dose-dependent manner.19,20 A normal QTc is less than or equal to 430 milliseconds for men and less than or equal to 450 milliseconds for women.21 As the QTc interval increases, the risk for life-threatening arrhythmia such as polymorphic ventricular tachycardia or TdP also increases. The risk of sudden cardiac death increases 4-fold when QTc is greater than 500 milliseconds.22
Evaluation for Clinical Eligibility
Once the clinician diagnoses OUD, he or she should obtain a comprehensive history, physical examination, and psychosocial evaluation, as well as initiate necessary tests and laboratory workup. During this session, the clinician should also ask about opioid withdrawal symptoms using the Clinical Opiate Withdrawal Scale (COWS).23 A score of 5 to 12 indicates mild withdrawal, 13 to 24 indicates moderate withdrawal, 25 to 36 indicates moderately severe withdrawal, and more than 36 indicates severe withdrawal.23
Comprehensive History
Methadone is a recommended treatment for patients with OUD who can give informed consent and have no specific contraindication for this treatment.12 Comprehensive history should include onset of opioid use, amount being used, frequency of use, mode of illicit drug use (e.g., oral, nasal, or intravenous route), and treatment history. It should also focus on individualized medical and behavioral risk evaluation to assess the risks and benefits of methadone, given its pharmacologic properties and adverse effect profile. This will require a comprehensive benefit-to-harm evaluation based on a thorough history, review of records, and physical examination.22 The clinician should ascertain the date and time of last use of any opioid and illicit drug.
Clinicians should inquire about medical and psychiatric histories and current medication list (both prescribed and over the counter), as well as check prescription monitoring programs. They should know prescribed controlled substances and interactions between methadone and other drugs that possess QTc prolonging properties or slow methadone elimination (Table 1).22 Clinicians must inform patients of arrhythmia risk when they intend to prescribe methadone and inquire about a history of arrhythmia, structural heart disease, and syncope as they develop the risk stratification and cardiac risk management plan (Table 2).12,22,25

Physical Examination
The prescribing clinician or another clinician on the team should conduct a comprehensive physical examination before he or she prescribes methadone. The examination should include identifying physical signs of opioid intoxication, such as reduced respiratory rate, drooping of the eyelids, and constricted pupils. When observing these physical signs, the clinician should initiate methadone to prevent an episode of opioid overdose. Before initiating methadone, however, the clinician should examine the patient for signs of opioid withdrawal, such as yawing, dilated pupils, lacrimation, rhinorrhea, abdominal cramps, nausea, and diarrhea, as well as COWS score at least in the mild withdrawal range (i.e., score of 5-12).23
Laboratory Tests and EKG Scans
Initial laboratory testing should include urine toxicology, comprehensive chemistry panel to detect electrolyte abnormalities, a complete blood cell count, liver enzyme screen, tests for hepatitis B and C, and HIV screen. Tests for sexually transmitted infections such as the rapid plasma regain (RPR) test to screen for syphilis should also be conducted.12
A pretreatment EKG scan should be completed. It is advisable not to start methadone treatment for patients with known QTc intervals higher than 500 milliseconds. The recommended initial dose of methadone ranges from 10 to 30 mg, with reassessment as clinically indicated (in 2 to 4 hours).12 A follow-up EKG within 30 days (during the titration phase) is advisable. By this time, most patients would have reached the 60 mg daily dose of methadone. Additional EKG scanning is recommended if the methadone dosage exceeds 120 mg/d or if patients have unexplained syncope or seizure during the course of methadone agonist therapy.22 For patients with a previous EKG scan indicating borderline QTc prolongation, repeat the follow-up scan in 4 to 7 days after initiation of methadone, then again in 30 days during the dose titration phase.
Clinicians should consider eliminating other risk factors and, if necessary, lower or taper the methadone dose if the QTc is more than or equal to 500 milliseconds during the course of treatment (Table 2).22,25 Clinicians, in consultation with patients, may need to consider the relative risk of adverse events due to QTc prolongation with methadone compared with the risk of morbidity and mortality of an untreated opioid use disorder.26
Consider changing to buprenorphine or naltrexone maintenance when risks of QTc prolongation are high, since these medications do not prolong the QTc.
Conclusions
OUD remains a significant cause of increased mortality and familial and economic burdens. Oral methadone, which is provided as a comprehensive treatment plan at state and federally designated opioid treatment programs, is an effective treatment and standard of care. A systematic approach involving comprehensive evaluation with history, physical examination, laboratory tests, and EKG scans helps formulate a cardiac risk management plan and continuous risk stratification plan. Results from these analyses determine the risk-benefit ratio, especially with respect to sudden cardiac death from arrhythmias precipitated by prolonged QTc.
References
- Institute of Medicine (US) Committee to Study Medication Development and Research at the National Institute on Drug Abuse; Fulco CE, Liverman CT, Earley LE, eds. Development of Medications for the Treatment of Opiate and Cocaine Addictions: Issues for the Government and Private Sector. National Academy Press; 1995. https://doi.org/10.17226/4906
- Sticherling C, Schaer BA, Ammann P, Maeder M, Osswald S. Methadone-induced Torsade de pointes tachycardias. Swiss Med Wkly. 2005;135(19-20):282-285. https://smw.ch/journalfile/view/article/ezm_smw/en/smw.2005.10939/2afcb02962894f9823d49e55ea95b4ecfa39e92d/smw_2005_10939.pdf/rsrc/jf
- America’s drug overdose epidemic: putting data to action. Centers for Disease Control and Prevention. Updated: December 16, 2020. Accessed: August 24, 2020. https://www.cdc.gov/injury/features/prescription-drug-overdose/index.html
- Opioid overdose. World Health Organization (WHO). Updated: August 28, 2020. Accessed: August 26, 2020. https://www.who.int/substance_abuse/information-sheet/en/
- Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, no 356. National Center for Health Statistics; 2020. https://www.cdc.gov/nchs/data/databriefs/db356-h.pdf
- Wilson, N, Kariisa M, Seth P, Smith IV H, Davis NL. Drug and opioid-involved overdose deaths—United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
- Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005. https://doi.org/10.1111/add.12863
- Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105(1-2):9-15. https://doi.org/10.1016/j.drugalcdep.2009.05.021
- Council of Economic Advisers. The full cost of the opioid crises: $2.5 trillion over four years. Press release. October 28, 2019. Accessed: August 26, 2020. https://www.presidency.ucsb.edu/documents/press-release-the-full-cost-the-opioid-crisis-25-trillion-over-four-years
- Opioid data analysis and resources. Centers for Disease Control and Prevention. Reviewed: March 19, 2020. Accessed: August 26, 2020. https://www.cdc.gov/drugoverdose/data/analysis.html
- Substance-related and addictive disorders. In: Schultz SK, Kuhl EA, eds. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Publishing; 2013:541-560.
- The American Society of Addiction Medicine (ASAM) national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. https://doi.org/10.1097/adm.0000000000000633
- Park TW, Cheng DM, Lloyd-Travaglini CA, Bernstein J, Palfai TP, Saitz R. Changes in health outcomes as a function of abstinence and reduction in illicit psychoactive drug use: a prospective study in primary care. Addiction. 2015;110(9):1476-1483. https://doi.org/10.1111/add.13020
- Matlock A, Allan N, Wills B, Kang C, Leikin JB. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clin Toxicol (Phila). 2011;49(6):443-447. https://doi.org/10.3109/15563650.2011.564585
- Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. US Food and Drug Administration. Published: October 2005. Accessed: February 12, 2021. https://www.fda.gov/media/71372/download
- Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354(9190):1625-1633. https://doi.org/10.1016/s0140-6736(99)02107-8
- Modesto-Lowe V, Brooks D, Petry N. Methadone deaths: risk factors in pain and addicted populations. J Gen Intern Med. 2010;25(4):305-309. https://doi.org/10.1007/s11606-009-1225-0
- Mujtaba S, Romero J, Taub CC. Methadone, QTc prolongation and torsades de pointes: Current concepts, management and a hidden twist in the tale? J Cardiovasc Dis Res. 2013;4(4):229-235. https://doi.org/10.1016/j.jcdr.2013.10.001
- Katchman AN, McGroary KA, Kilborn MJ, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther. 2002;303(2):688-694. https://doi.org/10.1124/jpet.102.038240
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013-1022. https://doi.org/10.1056/nejmra032426
- Cruciani RA. Methadone: to ECG or not to ECG...That is still the question. J Pain Symptom Manage. 2008;36(5):545-552. https://doi.org/10.1016/j.jpainsymman.2007.11.003
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MCP. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150(6):387-395. https://doi.org/10.7326/0003-4819-150-6-200903170-00103
- Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259. https://doi.org/10.1080/02791072.2003.10400007
- Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15(4):321-337. https://doi.org/10.1016/j.jpain.2014.01.494
- Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63. Publication No. PEP20-02-01-006. Substance Abuse and Mental Health Services Administration, 2020. https://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP20-02-01-006_508.pdf
- Cohen SP, Mao J. Concerns about consensus guidelines for QTc interval screening in methadone treatment. Ann Intern Med. 2009;151(3):216-219. https://doi.org/10.7326/0003-4819-151-3-200908040-00014


