Peer Reviewed
Eastern Equine Encephalitis
AUTHORS:
Paul J. Simpson II, MD; Saminder Singh Kalra, MD; Mwelwa Chizinga, MD; Cesar Trillo-Alvarez, MD; and Divya C. Patel, DO
AFFILIATION:
Division of Pulmonary, Critical Care, and Sleep Medicine, University of Florida, Gainesville, Florida
CITATION:
Simpson PJ, Singh Kalra S, Chizinga M, Trillo-Alvarez C, Patel DC. Eastern equine encephalitis. Consultant. 2022;62(2):e5-e7. doi:10.25270/con.2021.03.00007
Received September 22, 2020. Accepted January 18, 2021. Published online March 11, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Saminder Singh Kalra, MD, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Florida, P.O. Box 100225 JHMHC, Gainesville, FL 32610 (Saminder.kalra@medicine.ufl.edu)
A 68-year-old woman presented to our emergency department with chills, fatigue, and a sore throat of 1 week duration. Three days prior to presentation, her symptoms had progressed to generalized weakness and altered mental status.
History. She had a medical history significant for hypertension and labial herpes simplex virus (HSV) infection. She had never smoked, never consumed alcohol, and never used illicit drugs. She lived in a rural suburb of Florida and had recent exposures to mold, rats, and bats while renovating her cottage.
Physical examination. Upon presentation, her initial vital signs were taken: blood pressure, 200/80 mmHg; respiratory rate, 18 breaths/min; heart rate, 110 bpm, and temperature, 40.7 °C. The rest of the examination showed a nonverbal woman with a mild left facial droop and marked weakness of the left upper and lower extremities. There were several acute small fluctuant pustular wounds surrounded by mild erythema on the dorsal aspects of both hands (Figure 1). There were no bite marks, satellite lesions, or associated cellulitis.

Figure 1. Pustule on the patient’s right hand.
Diagnostic testing. Initial laboratory workup was notable for neutrophil-predominant leukocytosis with a level of 14.9 K/mm3. Otherwise, results of a comprehensive metabolic panel and urinalysis were normal, and results of a urine toxicology screen were negative. A chest radiograph returned normal results, and a computed tomography angiogram of head and neck showed no acute hemorrhage or perfusion abnormalities.
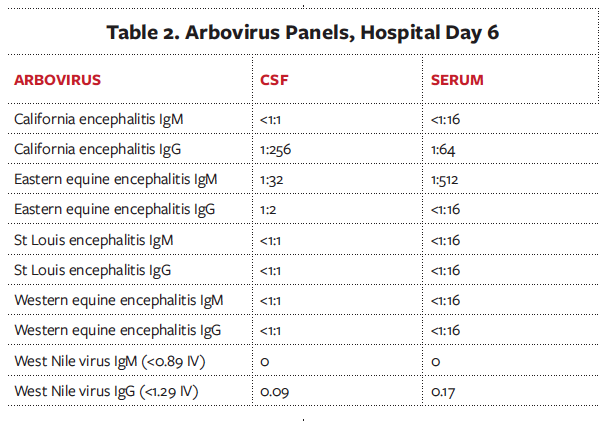
Shortly after she presented, the patient became lethargic, was intubated, and was empirically started on vancomycin, ceftriaxone, ampicillin, and acyclovir. A lumbar puncture was performed on hospital day 1 (Table 1). A cerebrospinal fluid (CSF) arboviral panel was only positive for California encephalitis immunoglobulin G (IgG) with 1:256 titer (Table 2). Results of a respiratory viral polymerase chain reaction (PCR) assay and HIV enzyme-linked immunosorbent assay (ELISA) were negative. An electroencephalogram (EEG) showed occasional high amplitude generalized periodic discharges and rare sharp temporal discharges. A magnetic resonance imaging (MRI) scan of the brain showed increased T2 signal in bilateral deep gray nuclei, striati, and thalami.

Her hospital course was complicated by nonconvulsive status epilepticus (NSCE) that was managed with multiple antiepileptics. Because her pustular wounds, history of bat exposure, and unresolved encephalopathy raised high clinical suspicion for rabies, serum, salivary, and skin samples were tested, results of which were negative. The skin lesion was sampled by needle aspiration, results of which were negative for bacterial, fungal and viral organisms.
Since our workup thus far had not hinted at a diagnosis and given the patient’s encephalopathy was not resolving, a repeat lumbar puncture was performed. One day later, the results were positive for the eastern equine encephalitis (EEE) immunoglobulin M (IgM) antibody in the CSF and serum.
Treatment and management. Antibiotics were immediately discontinued. Because of the severity of that patient’s neurologic dysfunction, treatment with intravenous immunoglobulins (IVIG) was initiated at 2 g/kg in 4 divided doses of 500 mg/kg/d. Over the next few days, the NSCE resolved, and her mental status started to improve. The patient underwent a tracheostomy and was transferred to a long-term acute care facility for ventilator weaning and further neurological recovery.
Discussion. EEE virus is a mosquito-borne alphavirus transmitted predominantly by Culiseta melanura, a mosquito that feeds primarily on birds and mammals.1 While most of the cases are asymptomatic or have mild prodromal symptoms, striking neurological deficits and impairment can be the first presenting symptoms in some patients. Human cases of EEE in the United States are predominantly localized in the Northeast and Gulf Coast regions, where warm weather and standing water allow rapid mosquito reproduction. This leads to a summer prevalence in the Northeast, but year-round disease in the Southeast United States. Even though less than 5% of EEE infections are associated with neurologic dysfunction, the degree of impairment is striking, and the mortality rate is high.1,2
Signs and symptoms of EEE are nonspecific and include fever, headaches, vomiting, and lethargy.2 However, once the neurological symptoms begin, the clinical picture can deteriorate quickly from altered mental status to obtundation, along with a high incidence of seizure and status epilepticus.2 Initial brain imaging is usually nonspecific, and MRI findings include hyperintense basal ganglia, thalami, and occasionally a linear density in the internal and external capsules called the “parenthesis sign,”3 which was seen in our patient (Figure 2). To make a definitive diagnosis of EEE, evaluations of clinical encephalitis and serum or CSF must show presence of viral nucleic acid or anti-EEE IgM or a 4-fold increase in the anti-EEE IgG level in serum from baseline.4 However, the delay of specific antibody production is a challenge when making a definitive diagnosis of EEE. According to one study,4 the average time from symptom onset to positive immunoglobulin titers was at least 6 days for serum and 9 days for CSF.
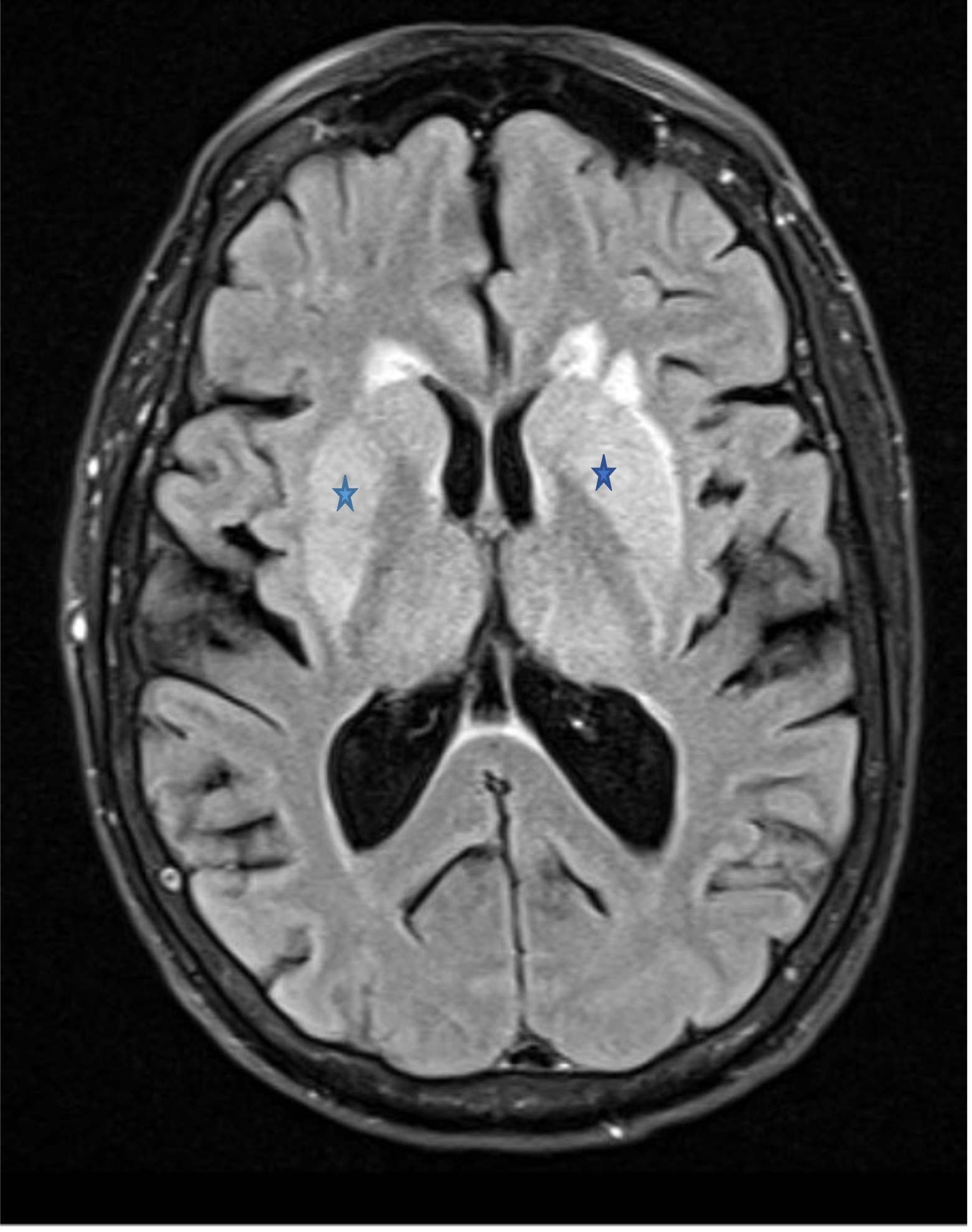
Figure 2. MRI scan of the brain show a bilateral “parenthesis sign” (marked by the stars).
Our patient presented with a fever, viral prodrome, and focal neurologic deficits with a background of significant environmental exposure. Given the high suspicion, a lumbar puncture was performed, and fluid analysis showed polycystic pleocytosis and an elevated protein level. A Gram stain was negative for organisms. Although, in our case, antibiotics were given before the lumbar puncture, which may have resulted in a false-negative. The high titer of California encephalitis IgG without a concordant increase in IgM reflected prior exposure to that virus. Results of an EEG with temporal discharges raised our suspicion for HSV encephalitis, although a brain MRI scan showed lesions in deep gray nuclei, strati, and thalami rather than the temporal lobe, which would be classic for HSV.
The differential diagnosis included viral encephalitides but also HSV and rabies, bacterial and fungal meningitis, and autoimmune encephalitis. However, the patient’s clinical presentation, geographical history, MRI findings, and epileptiform discharges on EEG strongly suggested arboviral encephalitis, despite initial negative lumbar puncture results. Thus, a repeat lumbar puncture was performed, which yielded the diagnosis.
The management of EEE remains primarily supportive. Corticosteroids have been suggested to reduce the inflammatory cascade but have not been systematically studied. IVIG theoretically downregulates antibody production, decreases cytokine production, and ultimately decreases T-cell activity. Several case studies have reported favorable neurologic recovery.5,6 This was our rationale for using IVIG in our case, although no trial has been conducted to test its efficacy. Sorafenib, a vascular endothelial growth factor receptor inhibitor, has also been shown to decrease viral replication in vitro and may reflect a potential future treatment.7
Prevention of EEE is primarily via mosquito control. There is an investigational new EEE vaccine, but it is given only in special circumstances and to certain populations, such as people working in diagnostic virology laboratories.8
Patient outcome. At her 3-month follow-up, the patient’s tracheostomy had been decannulated, but she continued to have memory deficits. The skin lesions on her hands had resolved with a residual faint scar and skin pigmentation.
References
- Armstrong PM, Andreadis TG. Eastern equine encephalitis virus--old enemy, new threat. N Engl J Med. 2013;368(18):1670-1673. https://doi.org/10.1056/nejmp1213696
- Lindsey NP, Staples JE, Fischer M. Eastern equine encephalitis virus in the United States, 2003-2016. Am J Trop Med Hyg. 2018;98(5):1472-1477. https://doi.org/10.4269/ajtmh.17-0927
- Nickerson JP, Kannabiran S, Burbank HN. MRI findings in eastern equine encephalitis: the “parenthesis” sign. Clin Imaging. 2016;40(2):222-223. https://doi.org/10.1016/j.clinimag.2015.10.012
- Sherwood JA, Brittain DC, Howard JJ, Oliver J. Antibody and viral nucleic acid testing of serum and cerebrospinal fluid for diagnosis of eastern equine encephalitis. J Clin Microbiol. 2015;53(8):2768-2772. https://doi.org/10.1128/jcm.00647-15
- Venkatesan A, Tunkel AR, Bloch KC, et al; International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-1128. https://doi.org/10.1093/cid/cit458
- Mukerji SS, Lam AD, Wilson MR. Eastern equine encephalitis treated with intravenous immunoglobulins. Neurohospitalist. 2016;6(1):29-31. https://doi.org/10.1177/1941874415578533
- Lundberg L, Brahms A, Hooper I, et al. Repurposed FDA-approved drug sorafenib reduces replication of Venezuelan equine encephalitis virus and other alphaviruses. Antiviral Res. 2018;157:57-67. https://doi.org/10.1016/j.antiviral.2018.07.005
- Hu WG, Steigerwald R, Kalla M, Volkmann A, Noll D, Nagata LP. Protective efficacy of monovalent and trivalent recombinant MVA-based vaccines against three encephalitis alphaviruses. Vaccine. 2018;36(34):5194-5203. https://doi.org/10.1016/j.vaccine.2018.06.064


