Peer Reviewed
A Man’s Sudden Cardiac Arrest: A Case of Brugada Syndrome
Authors
Ernest Oji Kanu, MD; Neilmegh Varada; Dustin Cheney, DO; and Kim Matz, DO
Citation
Kanu EO, Varada N, Cheney D, Matz K. A man’s sudden cardiac arrest: a case of Brugada syndrome [published online August 13, 2018]. Cardiology Consultant.
A 66-year-old man with a history of hypertension was brought to the emergency department (ED) by emergency medical services (EMS) after having experienced unwitnessed cardiac arrest. The man’s roommate had found him slumped over the steering wheel of his car and began chest compressions after calling EMS. On arrival, EMS personnel noted that the man was having ventricular fibrillation. He was shocked twice, resulting in restoration of sinus rhythm and a return of spontaneous circulation. He was intubated in the field and taken to the hospital.
On arrival to the ED, the patient was obtunded, with a blood pressure of 80/56 mm Hg, a heart rate of 91 beats/min, a respiratory rate of 18 breaths/min, a temperature of 35.9°C, and oxygen saturation of 80%. His Glasgow Coma Scale score was 3 of 15.
On physical examination, the chest was clear to auscultation. Heart sounds were normal, with regular rhythm. The abdomen was soft, with normal bowel sounds. He had no pitting edema of the lower extremities.
Diagnostic test results included the following values: white blood cell count, 12,520/µL (reference range, 3800-11,000/µL); hemoglobin 14.3 g/dL (reference range, 13.2-17.0 g/dL); platelet count, 243 × 103/µL (reference range, 150-400 × 103/µL); potassium, 3.2 mEq/L (reference range, 3.5-4.9 mEq/L); magnesium, 2.0 mEq/L (reference range, 1.7-2.4 mEq/L); and phosphorous, 4.3 mEq/L (reference range, 2.3-4.8 mEq/L). The levels of other serum electrolytes were normal. The cardiac troponin level was 0.06 ng/mL (reference range, 0.00-0.10 ng/mL), the creatine kinase (CK) level was 53 U/L (reference range, 55-400 U/L), the CK-MB isoenzyme level was 1.0 ng/mL (reference range, 0.5-3.6 ng/mL), and the ratio of CK-MB to CK (relative index) was 1.0.
A computed tomography scan of the head without contrast showed no acute intracranial abnormalities. An electrocardiogram (ECG) showed a right bundle branch block (RBBB) pattern with mild ST-segment elevations in leads V1 and V2 (Figure 1).

Hypothermic therapy was started. He received potassium along with intravenous fluids, was placed on a mechanical ventilator, and was transferred to the intensive care unit. Six hours later, the troponin level was 0.08 ng/mL, the CK-MB level was 34.1 ng/mL, the relative index was 0.9, and the CK level was 5970 U/L. ECG findings were unchanged. A heparin drip was started for non–ST-segment elevation myocardial infarction.
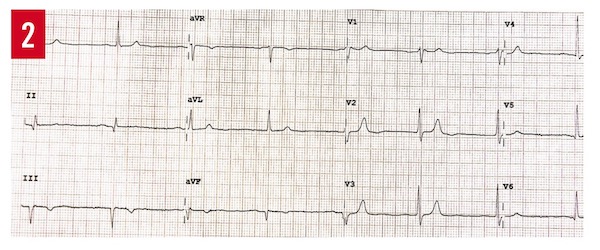
On hospital day 2, a transthoracic echocardiogram (TTE) showed a mildly reduced left-ventricular ejection fraction of 45% to 50% with no regional wall-motion abnormalities. Magnetic resonance imaging of the brain showed no acute intracranial abnormalities. The patient’s brother visited him and gave the additional history of their mother having died in her sleep at age 50, and that his brother had hypertension for which he had been taking lisinopril and hydrochlorothiazide. The patient’s brother also had been told some time ago that he had heart disease, of which he did not know the name. An ECG of brother was obtained, the results of which showed Brugada syndrome, type 3 pattern (Figure 2).
The patient was extubated on day 3. On day 4, a coronary angiogram revealed normal, patent coronary arteries. A left ventriculogram showed an ejection fraction of 60%. The heparin drip was stopped.
On day 5, an implantable cardioverter defibrillator (ICD) was placed, and on day 6, the patient was discharged to home, with a follow-up visit with the cardiology clinic scheduled for 1 week later. His brother was referred to see an electrophysiologist. Both men were counseled to avoid the use of drugs, alcohol, and other agents that might induce fatal arrhythmias in persons with Brugada syndrome.
DISCUSSION
Sudden cardiac arrest (SCA) is a sudden cessation of cardiac function that leads to hemodynamic collapse.1 Death is due to fast polymorphic tachycardia or ventricular fibrillation, if either is not terminated spontaneously or by intervention.1 There are cardiac and noncardiac causes for out-of-hospital SCA and sudden cardiac death (SCD). Most are due to ischemic heart disease (IHD).2 Fewer cases of SCA and/or SCD—approximately 5% to 10%—are due to arrhythmias occurring in a structurally normal heart, such as in Brugada syndrome.3
Brugada syndrome is named for Spanish cardiologists and brothers Pedro, Josep, and Ramon Brugada.4 In 1992, they described a distinct clinical syndrome of SCD occurring in patients with structurally normal heart and with the ECG characteristic of RBBB with ST-segment elevation in leads V1 through V3. The presence of this characteristic ECG finding in a person with no clinical symptoms of syncope or SCA is described as the Brugada pattern, of which there are 3 types (Figure 3).5 Type 1 has a coved-type ST elevation with a J point of least 2 mm (0.2 mv) and a gradually descending ST segment followed by a negative T wave. Type 2 has a saddleback shape with at least a 1-mm ST-segment elevation with a positive or biphasic T wave. Type 3 has either a coved or saddleback shape, with less than a 2-mm J-point elevation and less than a 1-mm ST-segment elevation.

The ECG pattern can be transient, and several factors can increase or reduce the ST elevation. Among the factors that can cause an elevation are the use of class Ia antiarrhythmic agents (eg, procainamide), class Ic antiarrhythmic agents (eg, flecainide, propafenone), class III antiarrhythmic agents (eg, amiodarone, sotalol), β-blockers, and calcium-channel blockers; fever; vagal stimulation; alcohol use; and cocaine use.6,7 Adrenergic stimulation as occurs with exercise reduces the ST elevation.
Brugada syndrome is genetically transmitted in an autosomal dominant pattern with variable penetrance,8 but it is a clinical diagnosis made when syncope or SCA occurs in a patient with a structurally normal heart and the typical ECG pattern with no other explicable cause.4 Death occurs through development of ventricular fibrillation if not terminated by way of intervention, such as with a defibrillator.1 ICDs have proven to be safe and effective in terminating life-threatening arrhythmias.9 If an ICD is not available, timely antiarrhythmic control with quinidine is an option.10
A clinical diagnosis of Brugada syndrome is made when other causes of SCA and/or SCD (such as IHD, cardiac structural abnormality, prolonged QT syndromes) have been excluded in a patient with the typical ECG findings of Brugada syndrome.4 Our patient’s TTE revealed no structural abnormality, and coronary angiography did not show any occlusive lesion in his coronary arteries. Based on his presentation of SCA, family history, ECG findings, normal TTE findings, and normal coronary angiography findings, he received a diagnosis of Brugada syndrome and was a candidate for ICD placement.
Current guidelines do not recommend ICD placement for this patient’s brother, who has the Brugada pattern on ECG but has had no clinical event.9 Further screening tools such as provocative pharmacologic testing5 and confirmation by genetic testing are available in some centers.8
Ernest Oji Kanu, MD, is a hospitalist and core faculty member at the Trios Health Internal Medicine Program and the Family Medicine Residency Program in Kennewick, Washington.
Neilmegh Varada is a fourth-year medical student at the Pacific Northwest University of Health Sciences in Yakima, Washington.
Dustin Cheney, DO, is a first-year resident in the Sollus Northwest Family Medicine Residency Program in Grandview, Washington.
Kim Matz, DO, is a second-year resident in the Trios Health Family Medicine Residency Program in Kennewick, Washington.
REFERENCES:
- Bayés de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117(1):151-159.
- Centers for Disease Control and Prevention (CDC). State-specific mortality from sudden cardiac Death—United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51(6):123-126.
- Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102(6):649-654.
- Brugada J, Brugada P, Brugada R. The syndrome of right bundle branch block ST segment elevation in V1 to V3 and sudden death—the Brugada syndrome. Europace. 1999;1(3):156-166.
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2(4):429-440.
- Kalavakunta JK, Bantu V, Tokala H, Kudenchery M. Sudden cause of cardiac death—be aware of me: a case report and short review on Brugada syndrome. Case Rep Med. 2010;2010:823490. doi:10.1155/2010/823490
- Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs.org). Heart Rhythm. 2009;6(9):1335-1341.
- Hedley PL, Jørgensen P, Schlamowitz S, et al. Brugada syndrome. Hum Mutat. 2009;30(9):1256-1266.
- Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited arrhythmia syndrome. Heart Rhythm. 2013;10(12):1932-1963.
- Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high risk patients with Brugada syndrome. Circulation. 2004;110(13):1731-1737.


